The flu season 2020/2021, all children aged two to three years on 31st August 2020 and all primary school children and those in year 7, (ages 4-11, not twelve years or older) on 31st August 2020, will be offered the flu vaccine. It will be also offered to children from six months of age who fit the clinical risk criteria.#

The flu vaccination for children is key to helping to lower the impact of flu by, providing direct protection of flu infection in children and by providing indirect protection, by lowering flu transmission transmissions from children, to other children, adults and those in at risk categories.
All children from the age of two are to be offered the Live attenuated influenza vaccine (LAIV) unless it is contraindicated. LAIV is an intranasal spray and has been shown to be more effective than inactivated influenza vaccines in children. As well as offering protection against the strains contained it in the vaccine, it may also offer protection against those that have undergone an antigenic drift. As the vaccine is live attenuated (weakened) it replicates natural infection which encourages improved immune memory, which in turn offers a better long-term protection to children.
It is unlikely that individuals cannot receive the flu vaccine, but when there are any concerns it is important to quickly, gather expert advice.
Contraindication to LAIV include an anaphylactic reaction to a previous flu vaccine, or to a component of the vaccine such as gelatine or a residue from the manufacturing process, gentamicin. LAIV should not be administered to any children who are clinically immunodeficient which is due to a condition or immunosuppressive therapy. It is also contraindicated in children receiving salicylate therapy because of the association of Reye’s syndrome. Any children known to be pregnant should not receive the LAIV version of the vaccine.
Children with asthma on inhaled corticosteroids regardless of the dose, can be given LAIV. Any children currently experiencing an acute exacerbation of symptoms including those that have had wheezing and or needed bronchodilator treatment in the past 72 hours, should not be given LAIV, but an inactivated vaccine alternative should be offered.
It is important to note that children with an egg allergy can be safely vaccinated with LAIV in any setting, children who have been admitted to intensive care for a previous anaphylaxis to egg should be given LAIV in a hospital setting. Those that have an egg allergy and another condition that contraindicates LAIV should be offered an inactivated flu vaccine with a low ovalbumin content. Children that are in a clinical risk group and aged under nine who have not been previously vaccinated against influenza will require a second dose whether given LAIV or and inactivated vaccine.
Ovalbumin content can be found at https://www.gov.uk/government/publications/influenza-vaccine-ovalbumin-content Where LAIV is contraindicated the inactivated vaccine should be offered instead.
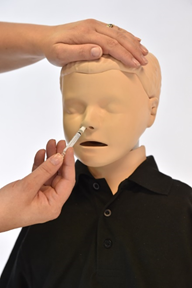
The live attenuated intranasal influenza vaccine (LAIV) is administrated using a nasal applicator which delivers 0.1ml of fluid into each nostril. As the plunger is depressed rapidly it convert fluid into a mist.
- To give the intranasal vaccine you must:
- Remove the applicator tip
- Do not remove the dose diver clip on the plunger
- Sit the patient upright and place just the tip inside one nostril
- Press the plunger until the dose divider prevents any further administration
- Remove syringe from nostril
- Remove the dose-divider clip
- Place the tip just inside the other nostril and depress plunger until none remains
- Discard the syringe as per your local guidance

Please refer to our website to explore all vaccination courses available https://ecgtraining.co.uk/all-courses/vaccination-courses/
Written by Lisa Humble ( ECG Clinical Development Manager), Wednesday 16th September 2020













